Tackling the fundamental physics behind inhalation for DDL2022
Just four weeks to go till DDL2022!
Cambridge, UK – 7th November 2022: We are counting down to the 2022 Drug Delivery to the Lungs Conference in Edinburgh. As proud sponsors and attendees of the coming event, we are pleased to announce that alongside our Quattrii™ high payload DPI platform we will also be demonstrating αeolus, a game changing product that is set to transform outdated inhaler technology and provide a significant and positive impact on clinical study outcomes – and the efficiency and consistency of drug delivery to patients.
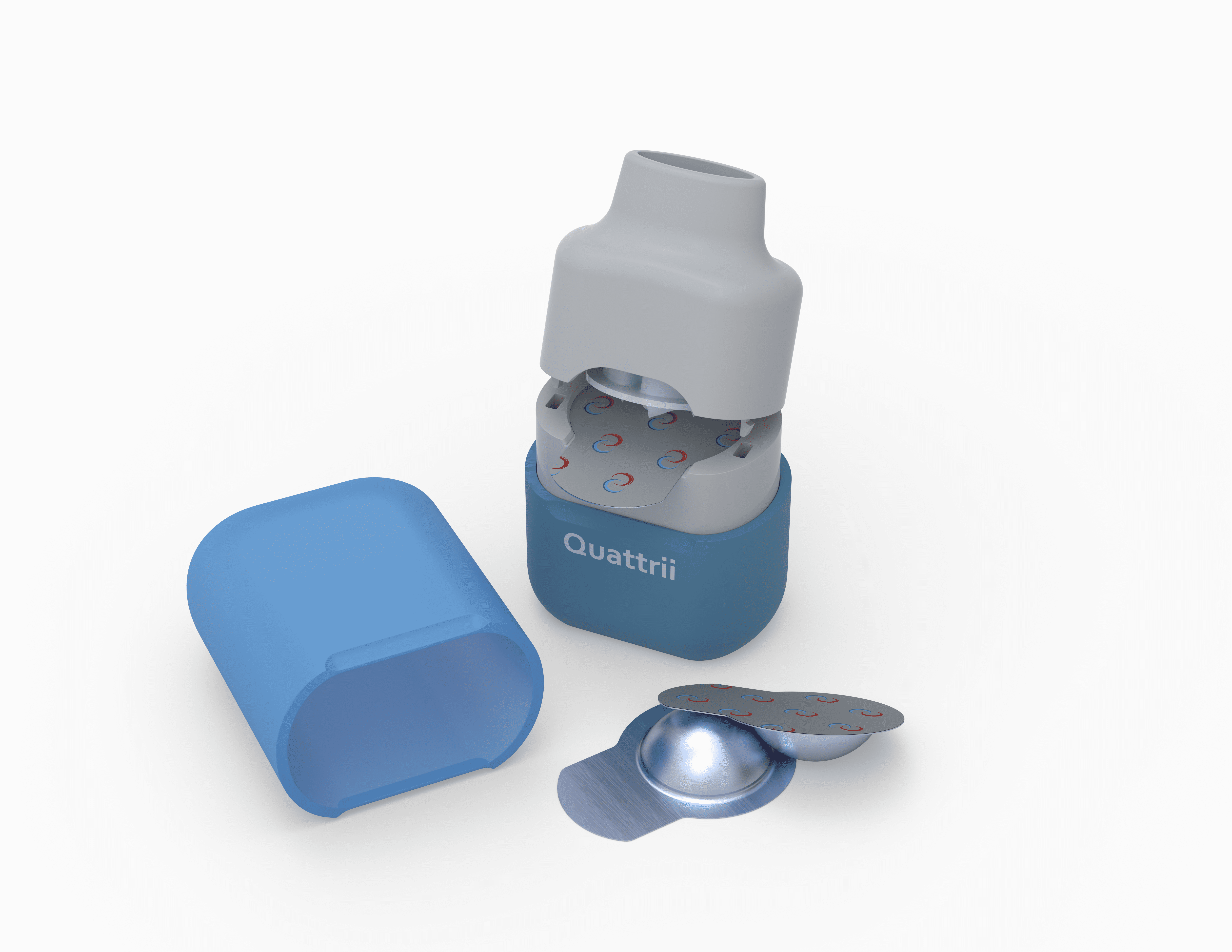

The brand new Quattrii™ high payload DPI from Cambridge Healthcare Innovations
You can come and see a demonstration of the new Quattrii DPI by booking a meeting with us here.
Our pioneering, new generation of inhaler technologies provides a solution to close the gap between the major global increase in chronic lung condition cases and antiquated inhaler technology that has lacked innovation since its invention in 1956.
Joan Soriano and colleagues … found that close to 545 million people in the world had a chronic respiratory disease in 2017, an increase of 39·8% since 1990.
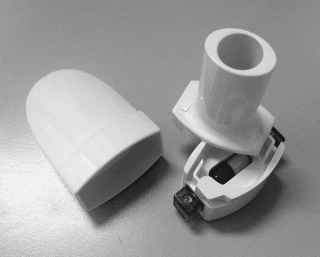




Above: the original RS01 “Monohaler” capsule DPI, circa. 1980s (left); the latest RS01 capsule DPI, 2022 (centre); CHI’s novel fluidic “gearbox”, the heart of the αeolus technology (right).
The ‘easier’ option leaves you gasping for air
Dry powder inhalers are not a new technology, yet current state-of-the-art inhalers deliver only 40-50% of the drug to the lung – and that is assuming the user inhales with sufficient effort and uses the device perfectly, which we know is not the case. The rest of the drug is wasted, landing in the mouth and throat causing unpleasant side effects.
Existing DPIs use the energy from the patient’s inhalation effort to work, and that level of effort (energy) is extremely variable, depending on the patients’ health, age and lung capability, with a healthy patient group differing by over an order of magnitude – even with the same training and instruction (Harris et al, Drug Delivery to the Lungs 21; pp79-87, Dec 9, 2010).
The underlying science of inhaled products is complex and designing a DPI that can deliver the same respirable dose with this huge range in patient effort and input energy is tough. As a result, the majority of DPIs entering the clinic unfortunately never reach the market.
“It’s wrong to assume that utilising existing inhaler technology is the easy option” states David Harris, Co-Founder and CTO of Cambridge Healthcare Innovations. “The proposition of using old, yet proven, DPI technology is understandably alluring even for the delivery of new molecules, but ultimately it places a significant burden on engineers to develop and optimise the drug formulation to achieve acceptable performance with their chosen DPI technology.
“It’s also dangerous to assume that just because a particular DPI has been accepted by the regulators with a previous drug molecule, it will achieve regulatory approval with a different, new drug molecule. Regulators are increasingly tightening standards, so using the same antiquated technology is certainly no guarantee of success.”
The approach mentioned above is all too common, resulting in a lack of innovation in the sector and making the regulatory submission process is by no means “easy”. The result is that most patients are using technology that was designed several decades ago, with limited effectiveness, and poor and often unintuitive usability. Pharmaceutical companies are often struggling to achieve success in the clinic, hobbled by inadequate device performance that fails to deliver sufficiently consistent dosing.
Join us in Edinburgh from 7th - 9th December
Come and see us at stand #104, and if you would like an in-depth discussion about our technology or your project, get in touch to pre-book a meeting
At Cambridge Healthcare Innovations, we have been tackling these challenges and the complex science behind inhalation head-on.
Whilst the fundamental physics of deagglomerating and aerosolising powder formulations is complicated, it is possible to substantially improve over the current state-of-the-art by adopting two simple principles:
- Maximise the energy transferred into the powder formulation throughout the duration of the inhalation event
- Optimise the type of energy that is transferred into the powder
The first principle is relatively straightforward to achieve – you simply need to extend the time window during which the powder is exposed to the input energy from the inhalation. Most inhalers emit their dose over a few hundred milliseconds, whereas users can inhale over several seconds. Designing an inhaler that can use all, or most of, the available time to work on the powder improves its effectiveness considerably.
The second principle, whilst simple to describe, is much more difficult to implement in practice. A patient will typically inhale a few grams of air with each inhalation – in contrast, the mass of a dose is usually only a few tens of milligrams. This huge imbalance means that the type of inhalation energy provided by the patient is not well suited to the task of breaking up tiny quantities of powder. Tiny quantities of powder are much more suited to comparable masses of air, providing this lower mass of air is at a significantly higher velocity. It is the pressure drop across the inhaler created by the user inhaling through it that determines the velocity of the airflow. The pressure drop depends on the diaphragm muscle strength of the patient, together with the impedance of their lungs and the airflow resistance of the inhaler. As we cannot realistically control the first two parameters, the only way to maximise the available pressure drop is to increase the resistance of the inhaler, such that it dominates over the effective resistance of their lungs. This approach will even out the playing field to some extent (diaphragm muscle strength is more consistent than lung impedance, see Harris et al, Drug Delivery to the Lungs 21; pp79-87, Dec 9, 2010) but is still ultimately subject to how strongly a patient can inhale.
At CHI we have been working for several years on a concept that breaks this rule. Using airflow alone we have created a fluidic “gearbox” that trades mass flowrate for an increase in differential pressure. This means that even patients with the weakest diaphragms and high lung impedance, who can barely activate a DPI breath-actuation mechanism (BAM), are able to use our devices effectively. The powder sees the amplified differential pressure, so even the weakest inhalations appear to the powder to be “normal”. This highly patented innovation results in a more inclusive device that can even successfully deliver reliever medication as an alternative to a pMDI (pressurised metered dose inhaler).
Ultimately, our ethos is to use our technology to help as many people as we can to manage their respiratory conditions, by successfully delivering new drugs to improve the lives of patients and in the process saving pharma companies effort, time and money. Join us on Stand #104 to find out more about our technology and how it can help deliver your new molecules. You can pre-book a meeting with us here.
