How do you deliver low potency molecules to the lung?
Join us at DDL2022 in Edinburgh from 7th - 9th December
Come and see us at stand #104, and if you would like an in-depth discussion about our technology or your project, get in touch to pre-book a meeting
TL;DR
Cambridge, UK – 25th November 2022: In the run up to DDL2022 we are talking about the limitations of existing DPI technologies at delivering high masses of low potency molecules to the lung and how our revolutionary Quattrii™ technology provides a solution.
We’re looking at the challenges faced in the pulmonary delivery of new low potency drug molecules, compared to the traditional, highly potent asthma and COPD drugs that almost all existing DPI technologies were designed to deliver.
With our Quattrii™ technology, the aluminium laminate (coldform) blister provides excellent moisture protection and the large (>1,400 µl) internal volume offers sufficient space to accommodate fill masses of up to 100 mg.
The innovative deagglomeration action of our Quattrii™ technology means that more drug is delivered to the deep lung, rather than to the mouth and throat of the patient. With high payloads, this significantly reduces the chance of cough reflex and minimises wastage of expensive drug product.
Ultimately, our ethos is to improve the lives of patients through the superior delivery of drugs, enabling better management of respiratory conditions, whilst minimising patient side-effects and drug wastage.
To find out how we can successfully deliver your drug molecules, book a meeting with us here at this year’s Drug Delivery to Lungs conference.
Addressing the delivery challenges of low potency molecules
The first pMDI was introduced in 1956, followed by the first capsule DPI in 1967. Since then, the variety of drugs delivered via the lungs has grown far beyond those for the treatment of conditions such as asthma and COPD that inhalers were originally designed for. However, these new molecules are often significantly less potent than those for asthma and COPD, and as a result require a greater dosage to achieve the desired therapeutic effect.
Traditionally, due to the tiny quantity of highly potent drug required for a dose, drugs delivered by DPIs are diluted with the inert sugar lactose, commonly referred to as the “carrier fraction” and typically making up at least 95% of the “fill mass”. A typical fill mass (the total mass of formulation filled for a single dose) for the treatment of respiratory illnesses such as asthma and COPD is approximately 10 mg. However, for many new molecules doses can be higher than 50 mg, which exceeds the limit of tolerability for many patients, and presents significant challenges for the current delivery technology, resulting in variability in the delivered dose and an undesirably low respirable fraction.
Our Quattrii™ DPI technology is designed specifically to deal with high masses of low potency drug molecules. Through its unique action, a highly respirable fraction is delivered over the first two seconds of inhalation, making the most effective use of the energy available and providing a consistent and highly respirable dose.
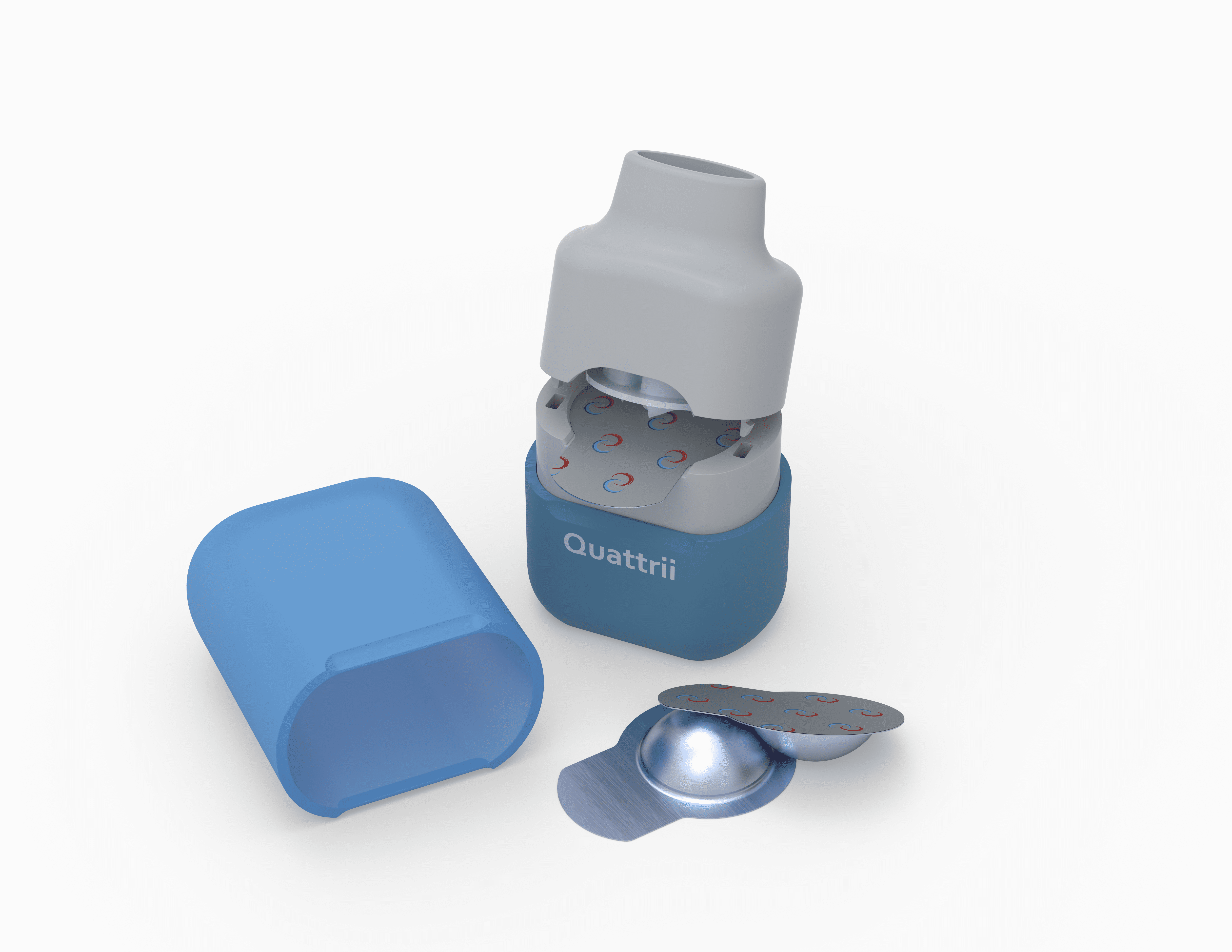

The brand new Quattrii™ high payload DPI from Cambridge Healthcare Innovations
A new solution to deliver high masses of drug effectively
The current solution to deliver large drug doses to the lung is for patients to take multiple inhalations to reach the required dose. With existing inhaler technology, going beyond one dose presents issues:
- A reservoir or multi unit-dose inhaler’s lifespan is reduced to less than a couple of weeks
- Human error leads to miscounting and incorrect drug dosage
- It’s time consuming and inconvenient, especially if it is necessary to load and unload multiple capsules to achieve a single, large dose
Another issue with high dose delivery to the lung is the cough reflex, which may be triggered both by the volume of powder landing in the mouth and throat and the rate with which it is delivered. While coughing is an important airway defence mechanism, it has obvious adverse effects on drug delivery, and so the formulation and drug delivery mechanism should be designed to avoid triggering it. However, existing inhalers deliver the bulk of the dose in a relatively short timeframe towards the beginning of the inhalation. This high flux of powder loading, combined with the high volume of powder for high dose delivery, can induce a cough in the patient.
Our Quattrii™ technology overcomes the above high-mass delivery challenges by:
- Only requiring a single blister per dose
- Only requiring a single inhalation per dose
- Minimising the delivery of non-respirable fraction, thereby substantially reducing mouth and throat deposition, and lowering the risk of cough
- Lengthening the delivery time period – i.e. over the first second or two of the inhalation, compared to just a few hundred milliseconds – thereby reducing the rate of delivery, again reducing the chance of triggering a cough reflex
Why not just modify existing, proven technology?
Existing DPIs were never designed to cope with such high masses of powder. Most inhaler devices today are designed to deliver limited amounts of formulation (between 3-25 mg) per dose:
- Reservoir DPIs have internal volumetric metering systems that rely upon the reservoir of powder formulation having a consistent bulk density to meter a precise mass of drug.
- Multi unit dose DPIs contain pre-metered blisters, that are “brim-filled”, meaning there’s no headroom above the dose in the blister, and the blister (and blister handling mechanisms) are designed for a specific dose size and formulation density.
Modification of these existing technologies to enable a larger dose of a new formulation is likely to require significant redesign, retooling and revalidation of the devices.
It must also be noted that the deagglomeration and aerosolisation mechanisms in devices initially designed for low powder volumes may be wholly unsuitable for high volume delivery, resulting in a drop-off in performance and drug wastage. If your molecule is a biologic, it may be extremely expensive to produce, so any form of wastage is highly undesirable – whether it’s lost in the mouth and throat, or the upper airways of the patient, or retained within the primary container of the inhaler device.
Our developments for effective drug delivery
Quattrii™ DPI Technology
For early-stage exploration of dry powder delivery, a typical “go-to” device is an existing capsule dry powder inhaler (cDPI), such as the Berry RS01. These are designed to work with the industry-standard size 2 and 3 capsules. cDPIs achieve deagglomeration and aerosolisation of the powder by spinning, rattling, or agitating the capsule itself. They can handle a range of dose sizes, however if increasing the size of the capsule is necessary for a high-volume, low potency drug, the whole inhaler is likely to require redesign.
The Quattrii™ DPI technology has been designed to overcome this problem. Scaling the dose up or down, according to the requirements of upcoming new molecules, only necessitates a change to our primary pack – the blister – without changing any other aspect of the device. The Quattrii™ technology works with a range of blister sizes – and each blister size can handle a range of fill masses.
Our coldform blisters packs
Another issue with cDPIs is that the drug’s primary packaging – the capsule – offers zero moisture protection, meaning hygroscopic formulations need to be packaged in secondary (moisture-proof) containers to achieve adequate shelf life.
Quattrii™ avoids this complexity by using a coldform blister as the primary pack, removing the requirement for secondary packaging, providing inherent moisture protection right up to the moment of use and significantly improving usability.
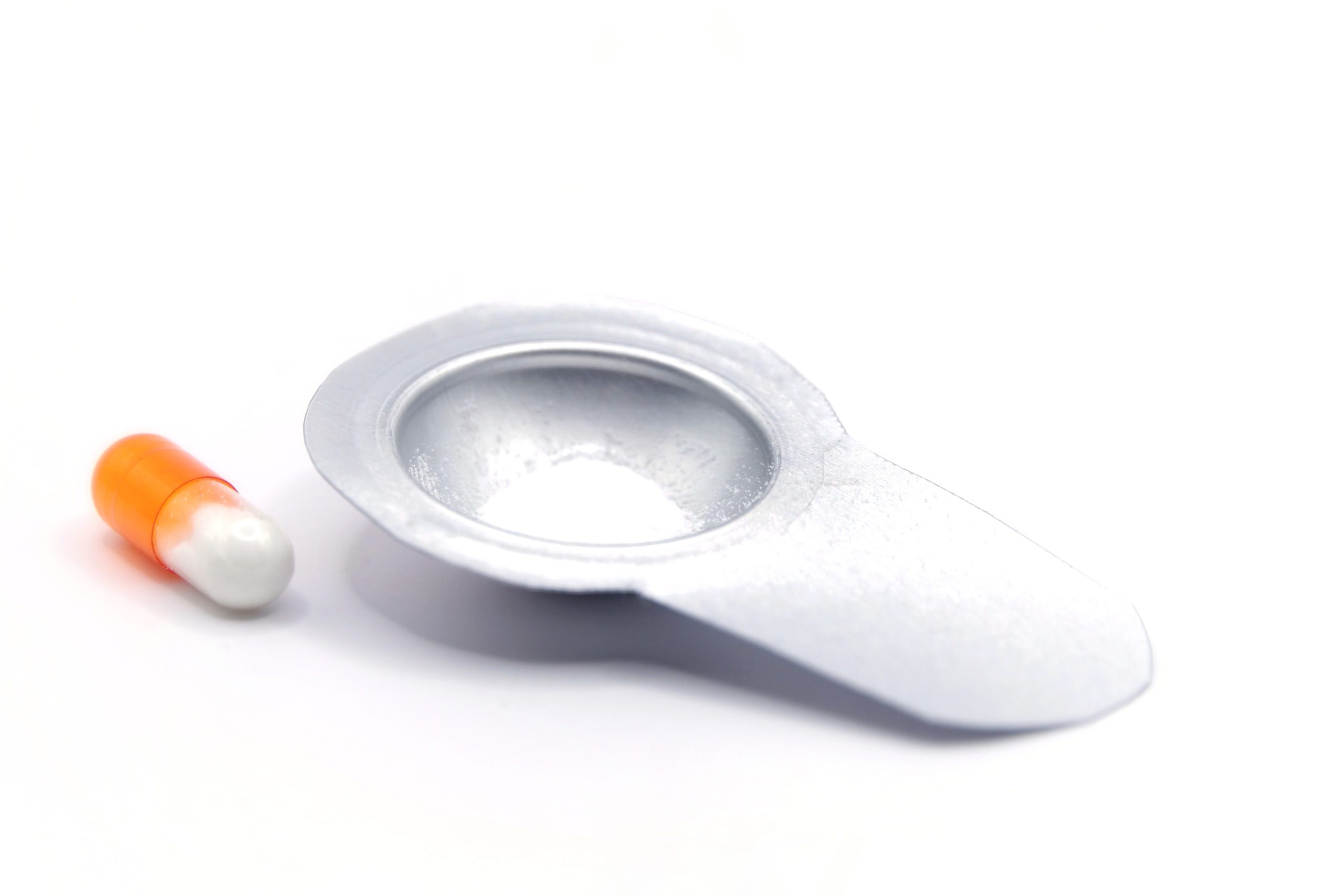

100 mg of powder in a size 3 capsule (left) and in a Quattrii™ blister (right, lid foil removed). The Quattrii™ blister can be larger or smaller, depending on the dosage requirements.
Ultimately, our ethos is to improve the lives of patients through the superior delivery of drugs, enabling better management of respiratory conditions, whilst minimising patient side-effects and drug wastage. Book a meeting with us here to find out how our Quattrii™ technology can help delivery your drug.
