Ready to test your formulation in a high-performance DPI?
We are excited about the performance we’re achieving.
If you’d like to see how well αeolus can deliver your formulation, just get in touch.
Cambridge, UK – 18th July 2022: In collaboration with Kindeva, over the past year we have been working hard developing our dry powder inhaler (DPI) platform technology – αeolus – for a wide range of formulations. Now, following the promising early results we published at RDD 2022, we are pleased to be able to share further encouraging data from the development of this novel platform technology.
Most DPIs on the market typically deliver around 10 to 20 mg of powder – however, there is increasing interest in high-dose delivery for a range of upcoming therapies. So we have been benchmarking the performance of our latest αeolus designs to the globally successful RS01 device, with doses ranging from the ‘typical’ right up into state of the art higher fill mass territory.
We tested with 12.5 mg, 50 mg and 100 mg of salbutamol sulphate and lactose harvested from Ventolin Accuhaler, testing αeolus alongside Berry’s ultra-high resistance version of the RS01 Monodose inhaler (size 3 capsule). To put those fill weights in perspective, 100 mg is roughly eight doses of Ventolin Accuhaler in a single inhalation. Testing was conducted in vitro using the Next Generation Impactor.
In this article:
- World class performance with reduced side effects
- Consistent high fill mass capability
- Broader access to therapies
- Increasing patient comfort and adherence
- Versatility across the fill-mass range
World class performance with reduced side effects
Typically, DPIs deposit 40-70% of the drug into the patient’s mouth and throat – this is a significant, undesirable issue that can lead to unpleasant side effects such as candidiasis.
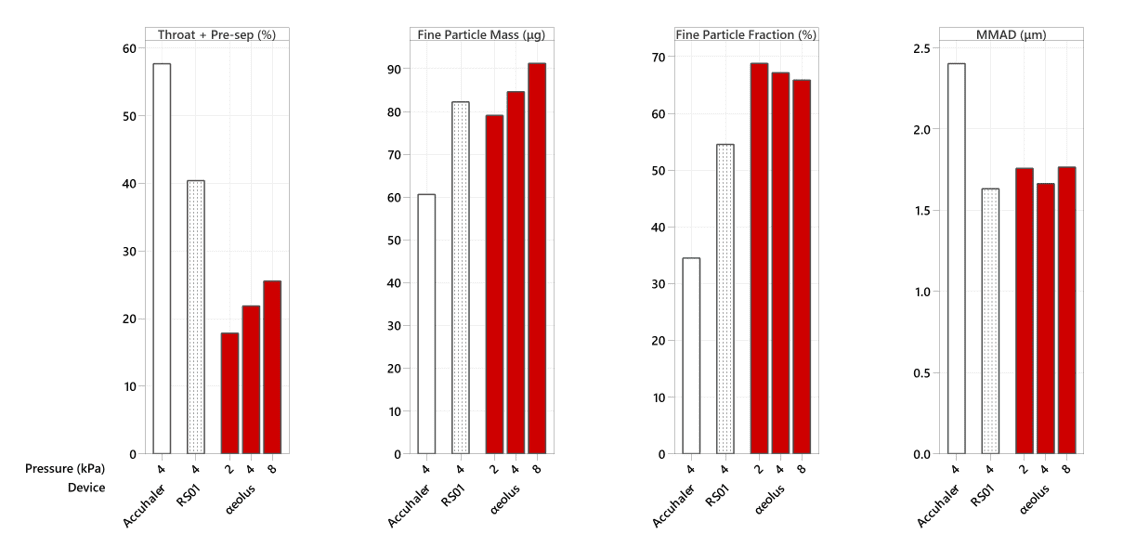
In our testing with a ‘typical’ fill mass of 12.5 mg, RS01 deposited 40% of its drug into the mouth and throat, and Ventolin Accuhaler 57%. However, for the same inhalation effort and with the same amount of drug reaching the deep lung, αeolus only deposited 21% into the mouth and throat.
That’s half the mouth and throat deposition of RS01 and a third of that of Ventolin Accuhaler.
This is due to αeolus having a world class Fine Particle Fraction of 65 – 70% (percentage of the emitted dose that is small enough to reach the deep lung).
So, if you are looking for a DPI for your drug product and want:
- The high moisture protection offered by doses in individual foil blisters
- A high fine particle mass with considerably lower mouth and throat deposition
- A highly customisable device
αeolus may be the right technology for you.
12.5 mg fill mass
Figure 1: Throat + Pre-Separator deposition (as a percentage of total recovered), Fine Particle Mass (FPM), Fine Particle Fraction (FPF), and Mass Median Aerodynamic Diameter (MMAD) profile of αeolus versus Ventolin Accuhaler, Berry’s ultra-high resistance RS01 Monodose at 2 kPa (αeolus only), 4 kPa and 8 kPa (αeolus only) mouth pressure using 12.5 mg of Ventolin salbutamol sulphate formulation.
Consistent high fill mass capability
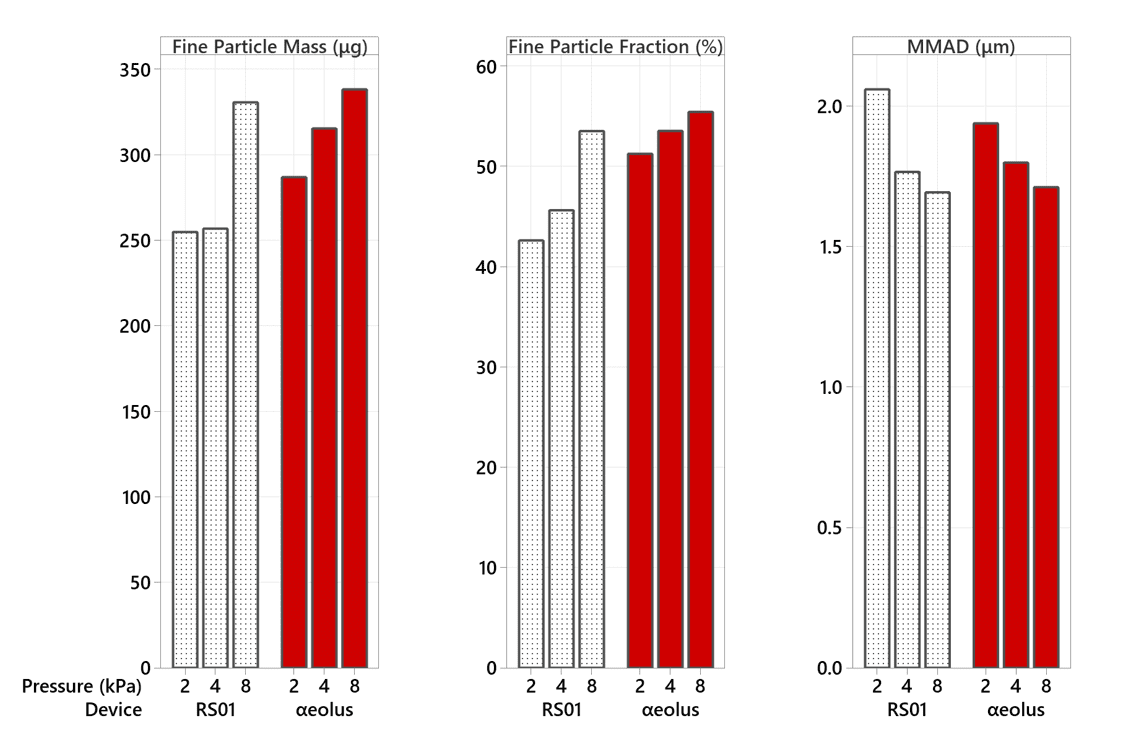
At both 50 mg and 100 mg fill masses, αeolus delivers a higher Fine Particle Mass and Fine Particle Fraction at a mouth pressure of 2 kPa (half the standard in vitro test pressure) than RS01 at 4 kPa (see Figures 1 and 2).
This indicates that, even with half the inspiratory effort, αeolus can consistently deliver a high dose of respirable drug to the deep lung.
Broader access to therapies
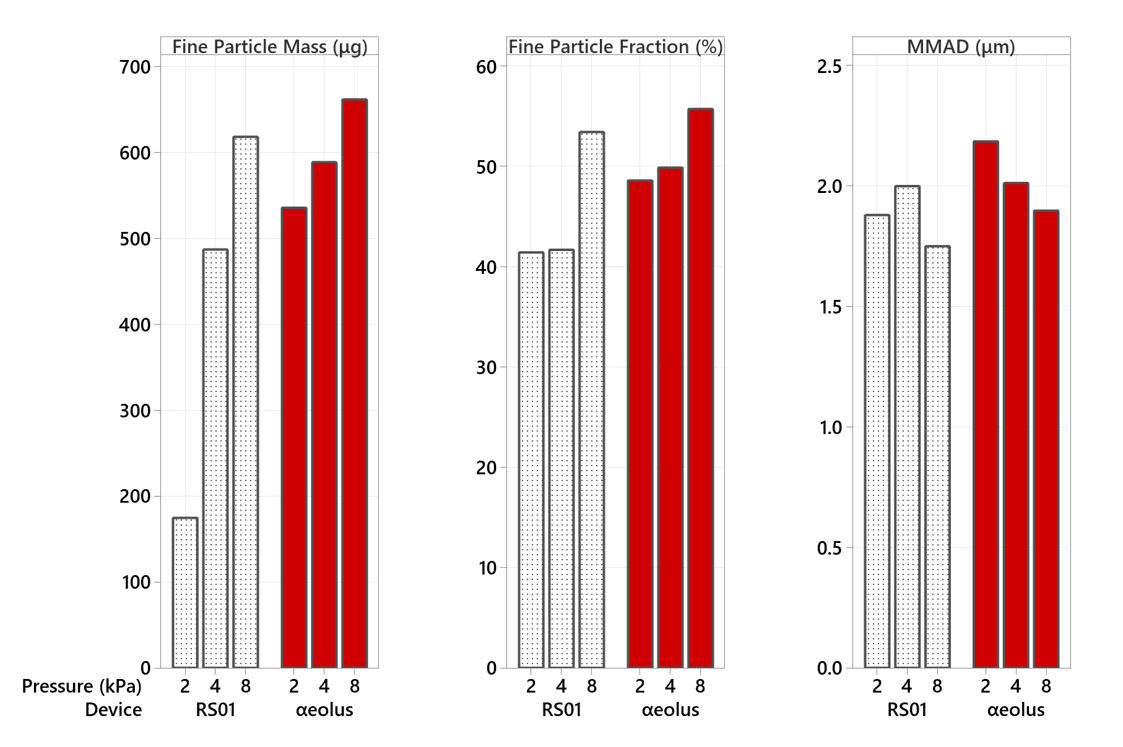
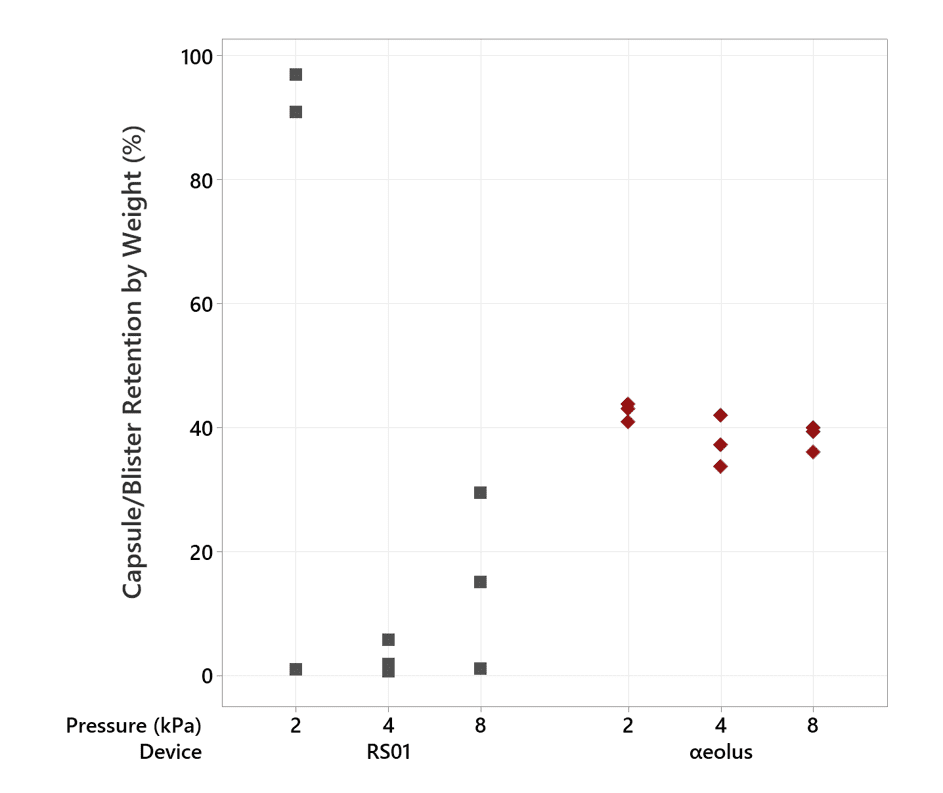
In our 100 mg testing at 2 kPa, the RS01 device failed to evacuate its capsule two out of three times – this is reflected in the poor Fine Particle Mass result at 2 kPa (and can be seen more clearly in Figure 4). αeolus continues to perform consistently well, even at this low mouth pressure and high fill-mass.
This brings the possibility of high payload DPI treatments for those with lower inspiratory strength, for whom previously a DPI may not have been suitable.
Increasing patient comfort and adherence
Many formulations, such as the Ventolin used in this study, are carrier-based: they combine a small amount of fine Active Pharmaceutical Ingredient (API), in this case salbutamol, attached to much coarser ‘carrier’ particles, in this case lactose. The carrier particles are there to improve the flowability of the powder and make it easier to handle during manufacture. The device needs to separate the API from the carrier for the API to be able to reach the lung and not to deposit in the mouth and throat with the lactose carrier particles.
For the sake of patient comfort and treatment tolerability when delivering high payloads, it would be beneficial for the device to not only separate the API from the carrier, but to prevent at least a proportion of the carrier particles from leaving the device in the first place. This is what αeolus is designed to do: to preferentially release the respirable fine powder fraction while retaining a proportion of the non-respirable carrier fraction.
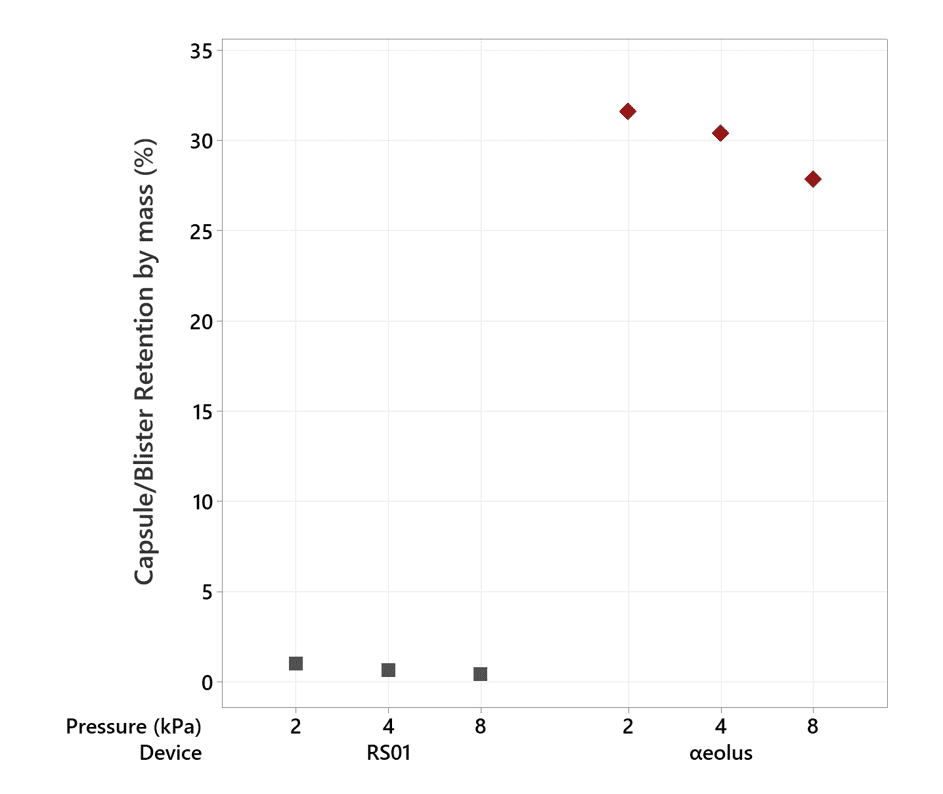
Not only is αeolus delivering more drug, it is also holding back approximately 30% of the lactose in the blister at 50 mg and 40% at 100 mg (see Figures 4 and 5), meaning that this unwanted powder won’t be landing in the user’s mouth and throat, potentially improving patient comfort.
Versatility across the fill-mass range
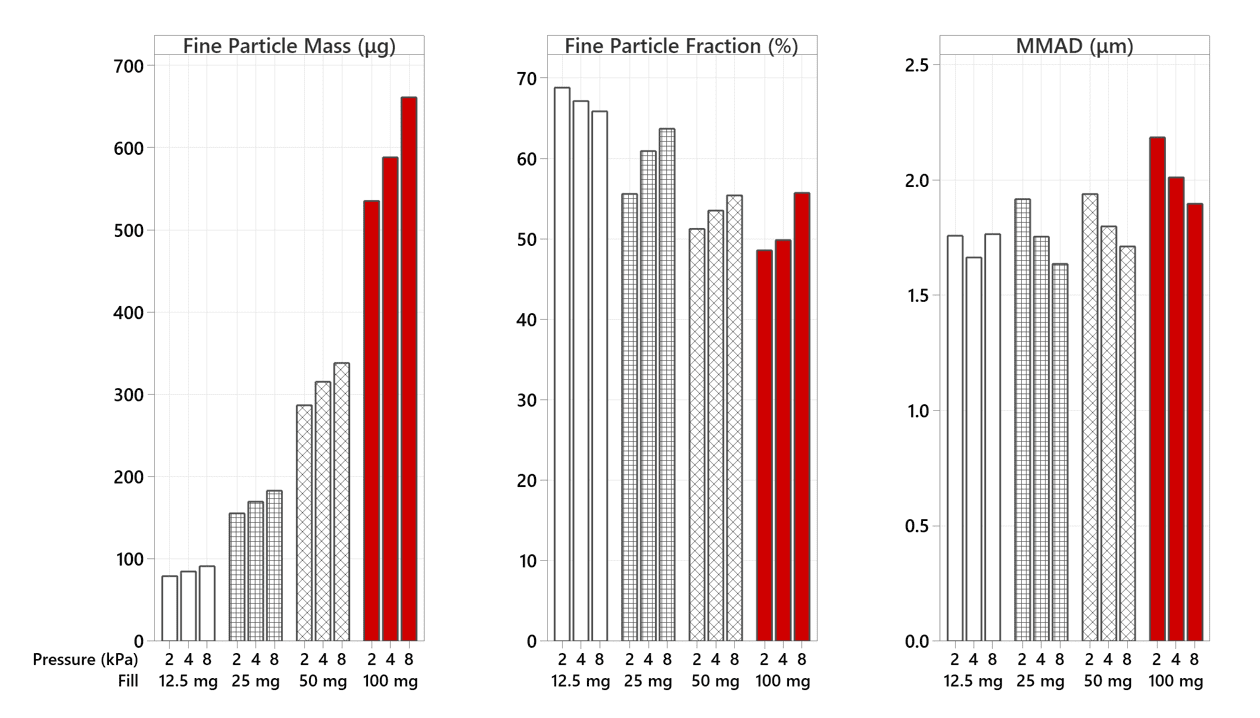
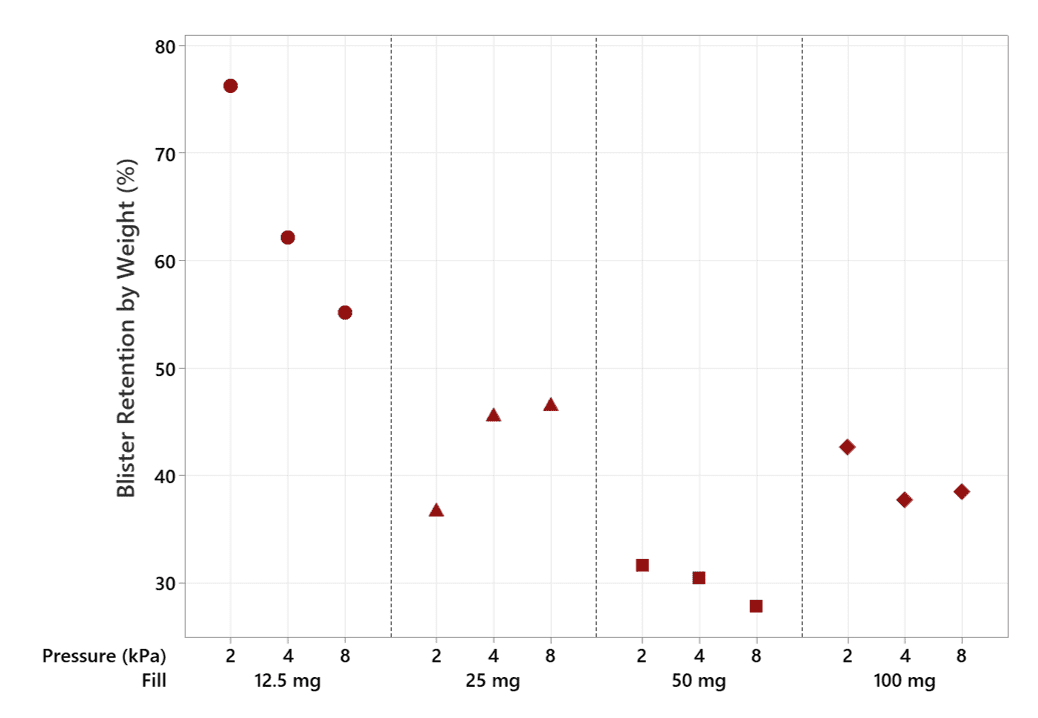
The FPF improves as the fill-mass reduces (Figure 6), augmented by αeolus preventing a high proportion of the unwanted lactose carrier from being inhaled by the user (Figure 7).
Figure 6: Fine Particle Mass (FPM), Fine Particle Fraction (FPF), and Mass Median Aerodynamic Diameter (MMAD) profile of αeolus delivering 12.5 mg, 25 mg, 50 mg and 100 mg of Ventolin harvested formulation at 2, 4, and 8 kPa mouth pressures.
Figure 7: Mean blister retention by weight, as a percentage of fill mass, for αeolus, at 2, 4, and 8 kPa mouth pressure using Ventolin harvested formulation.
We’re ready to test your formulation
We’re highly encouraged by these results. Not only that, but in the creation of these data, our αeolus bench test rigs have been honed and optimised to be ‘plug-and-play’, so, if you are interested in seeing what αeolus can do with your formulation, just get in touch.